- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
-
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
-
J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
-
DOI: https://doi.org/10.17998/jlc.2023.12.25
-
-
1,029
Views
-
134
Downloads
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material
- Background/Aim
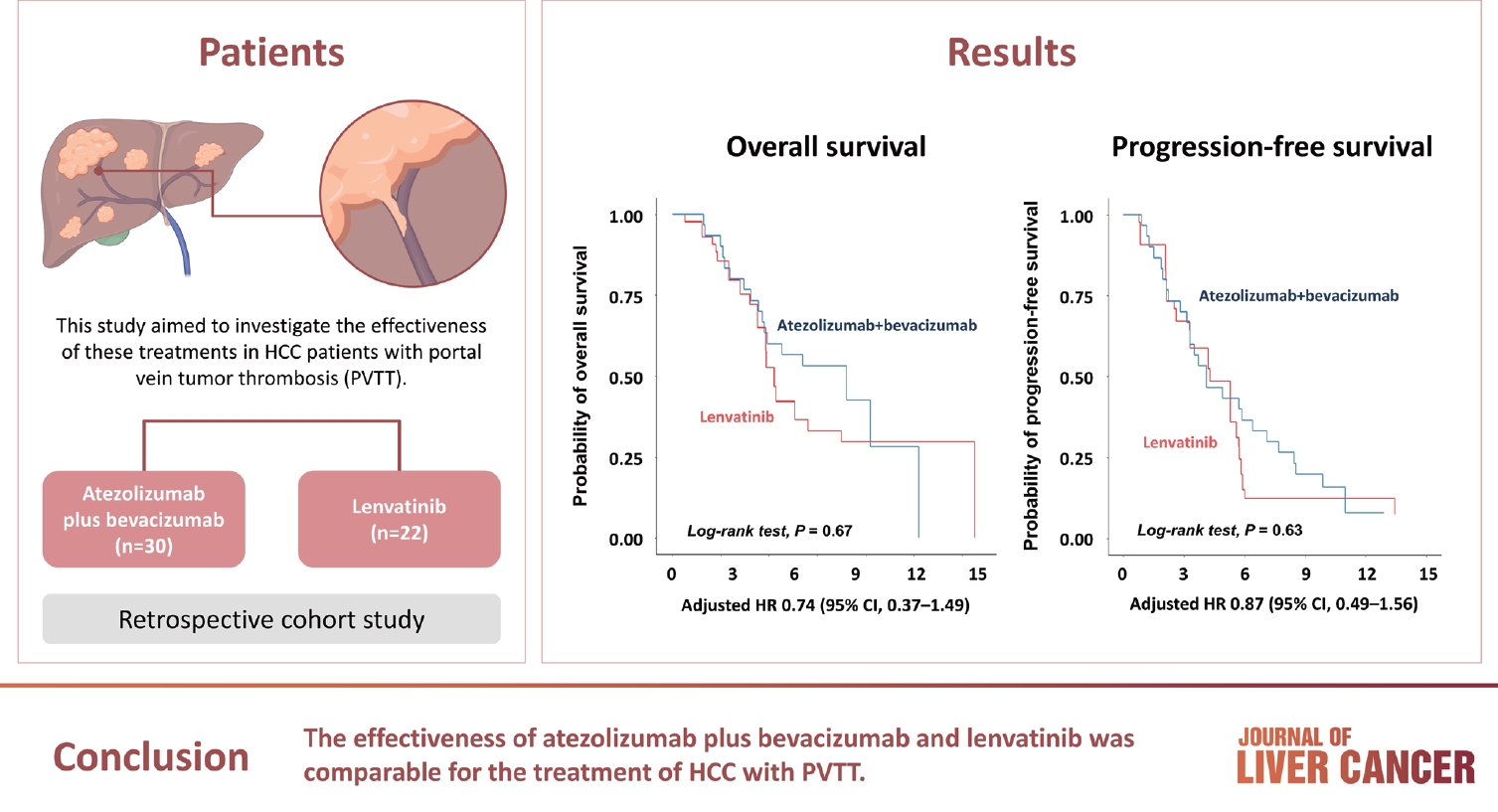
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.
- Use of doxorubicin-eluting bead transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein invasion: a prospective study
-
Su Jong Yu, Yun Bin Lee, Eun Ju Cho, Jeong-Hoon Lee, Hyo-Cheol Kim, Jin Wook Chung, Jung-Hwan Yoon, Yoon Jun Kim
-
J Liver Cancer. 2023;23(1):166-176. Published online March 3, 2023
-
DOI: https://doi.org/10.17998/jlc.2023.02.08
-
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material
- Background/Aim
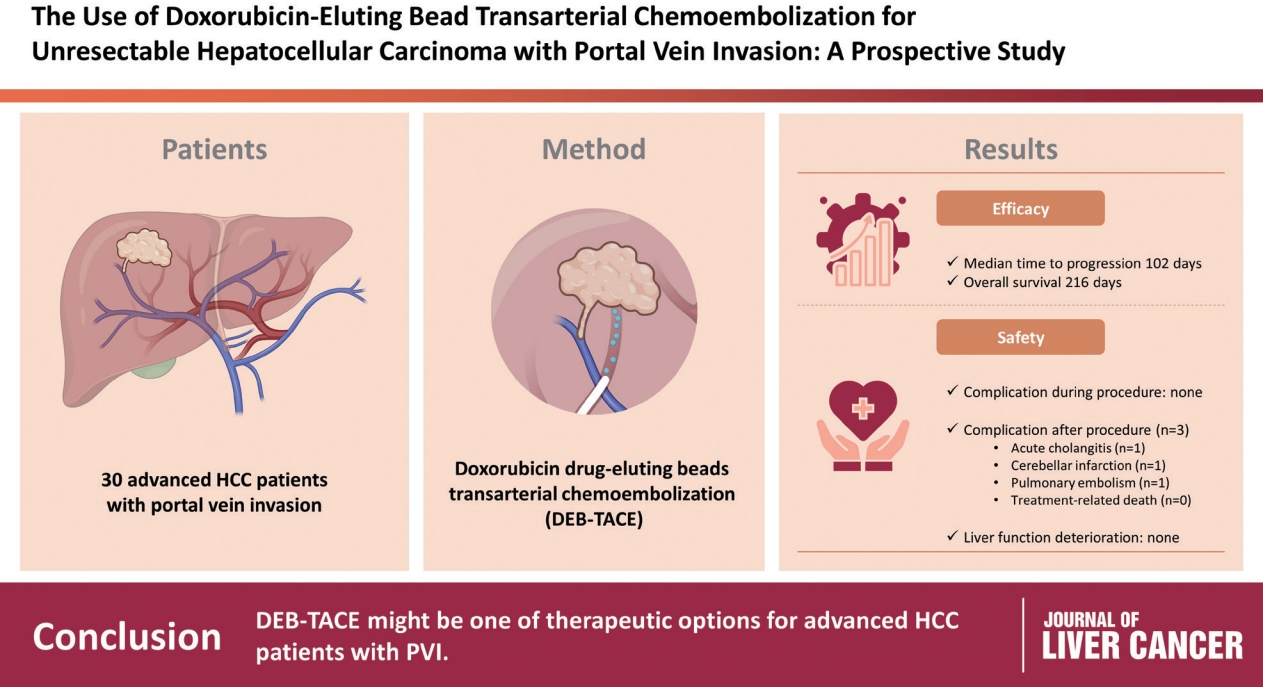
To evaluate the applicability of transarterial chemoembolization (TACE) treatment with doxorubicin drug-eluting beads (DEBs) in advanced hepatocellular carcinoma (HCC) patients with portal vein invasion (PVI).
Methods
This prospective study was approved by the institutional review board and informed consent was obtained from all participants. A total of 30 HCC patients with PVI received DEB-TACE between 2015 and 2018. The following parameters were evaluated: complications during DEB-TACE, abdominal pain, fever, and laboratory outcomes, including liver function change. Overall survival (OS), time to progression (TTP), and adverse events were also analyzed and assessed.
Results
DEBs measuring 100–300 μm in diameter were loaded with doxorubicin (150 mg per procedure). There were no complications during DEB-TACE and no significant differences in the levels of prothrombin time, serum albumin, or total bilirubin at follow-up compared to baseline. The median TTP was 102 days (95% confidence interval [CI], 42–207 days) and the median OS was 216 days (95% CI, 160–336 days). Three patients (10%) had severe adverse reactions, including transient acute cholangitis (n=1), cerebellar infarction (n=1), and pulmonary embolism (n=1), but no treatment-related death occurred.
Conclusions
DEB-TACE may be a therapeutic option for advanced HCC patients with PVI.
- The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: a systematic review and meta-analysis
-
Young Chang, Soung Won Jeong, Jae Young Jang, Hyuksoo Eun, Young‑Sun Lee, Do Seon Song, Su Jong Yu, Sae Hwan Lee, Won Kim, Hyun Woong Lee, Sang Gyune Kim, Seongho Ryu, Suyeon Park
-
J Liver Cancer. 2022;22(2):167-177. Published online September 29, 2022
-
DOI: https://doi.org/10.17998/jlc.2022.09.19
-
-
2,603
Views
-
73
Downloads
-
1
Citation
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material
- Background/Aim
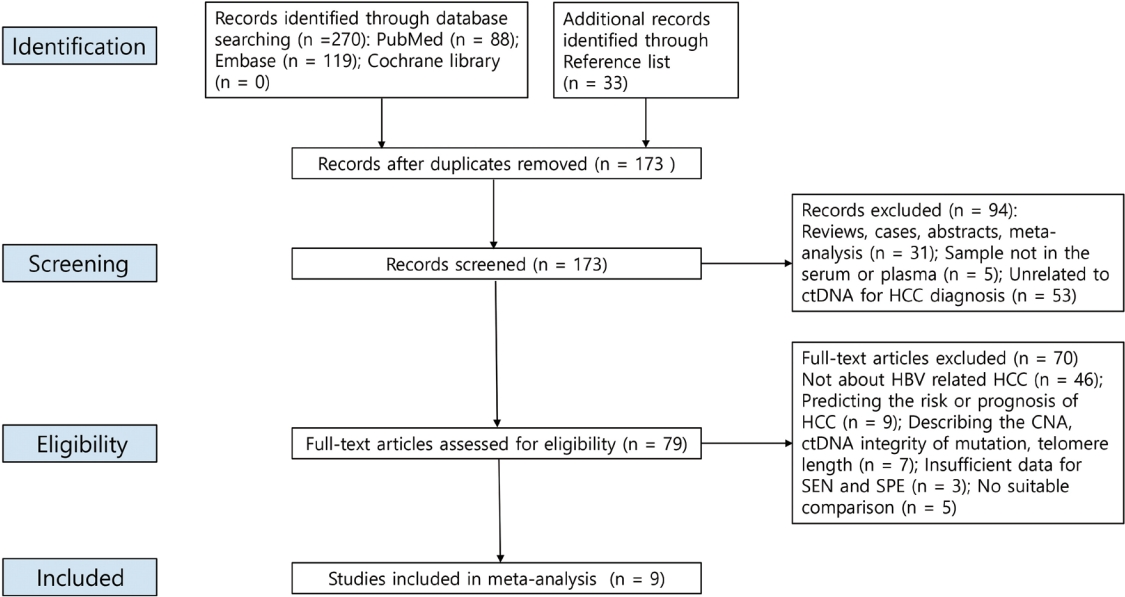
New biomarkers are urgently needed to aid in the diagnosis of early stage hepatocellular carcinoma (HCC). We performed a meta-analysis on the diagnostic utility of circulating tumor DNA (ctDNA) levels in patients with hepatitis B virus-induced HCC.
Methods
We retrieved relevant articles from PubMed, Embase, and the Cochrane Library up to February 8, 2022. Two subgroups were defined; one subset of studies analyzed the ctDNA methylation status, and the other subset combined tumor markers and ctDNA assays. Pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC) were analyzed.
Results
Nine articles including 2,161 participants were included. The overall SEN and SPE were 0.705 (95% confidence interval [CI], 0.629-0.771) and 0.833 (95% CI, 0.769-0.882), respectively. The DOR, PLR, and NLR were 11.759 (95% CI, 7.982-17.322), 4.285 (95% CI, 3.098- 5.925), and 0.336 (0.301-0.366), respectively. The ctDNA assay subset exhibited an AUC of 0.835. The AUC of the combined tumor marker and ctDNA assay was 0.848, with an SEN of 0.761 (95% CI, 0.659-0.839) and an SPE of 0.828 (95% CI, 0.692-0.911).
Conclusions
Circulating tumor DNA has promising diagnostic potential for HCC. It can serve as an auxiliary tool for HCC screening and detection, especially when combined with tumor markers.
-
Citations
Citations to this article as recorded by  - 16S rRNA Next-Generation Sequencing May Not Be Useful for Examining Suspected Cases of Spontaneous Bacterial Peritonitis
Chan Jin Yang, Ju Sun Song, Jeong-Ju Yoo, Keun Woo Park, Jina Yun, Sang Gyune Kim, Young Seok Kim
Medicina.2024; 60(2): 289. CrossRef
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
-
Min Kyung Park, Su Jong Yu
-
J Liver Cancer. 2022;22(1):69-74. Published online March 21, 2022
-
DOI: https://doi.org/10.17998/jlc.2022.03.09
-
-
3,277
Views
-
106
Downloads
-
4
Citations
-
 Abstract Abstract
 PDF PDF
- Treatment options for advanced hepatocellular carcinoma (HCC) have been rapidly evolving. Herein, we describe a patient with advanced HCC and portal vein tumor thrombosis (PVTT) who responded decisively to a multidisciplinary approach. The patient had an ill-defined infiltrative HCC (diffuse subtype), with several intrahepatic metastasis and tumor invasion of left portal vein. Concurrent use of transarterial radioembolization (TARE) and systemic therapeutics (atezolizumab + bevacizumab) ultimately proved successful. There was marked reduction in tumor volume after TARE and an additional three cycles of atezolizumab plus bevacizumab. This concurrent treatment was well tolerated, without adverse events during immunotherapy. The impressive results achieved suggest that concurrent TARE and combination atezolizumab/bevacizumab is a promising treatment approach for advanced HCC with PVTT.
-
Citations
Citations to this article as recorded by  - Biologics, Immunotherapies, and Cytotoxic Chemotherapy for Hepatocellular Carcinoma following Current Recommendations by the BCLC: A Review of Agents
Rajangad S. Gurtatta, Sydney E. Whalen, Charles E. Ray
Seminars in Interventional Radiology.2024; 41(01): 084. CrossRef - Combining immunotherapy with transarterial radioembolization
ZeynepCeren Balaban Genc, Efe Soydemır, SevalAy Ersoy, Tunc Ones
Indian Journal of Nuclear Medicine.2023; 38(2): 145. CrossRef - The New Era of Systemic Treatment for Hepatocellular Carcinoma: From the First Line to the Optimal Sequence
Maria Cerreto, Ferdinando Cardone, Lucia Cerrito, Leonardo Stella, Francesco Santopaolo, Maria Pallozzi, Antonio Gasbarrini, Francesca Romana Ponziani
Current Oncology.2023; 30(10): 8774. CrossRef - Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus?
Won Hyeok Choe
Journal of Liver Cancer.2022; 22(1): 1. CrossRef
- Deciphering and Reversing Immunosuppressive Cells in the Treatment of Hepatocellular Carcinoma
-
Su Jong Yu, Tim F. Greten
-
J Liver Cancer. 2020;20(1):1-16. Published online March 31, 2020
-
DOI: https://doi.org/10.17998/jlc.20.1.1
-
-
6,811
Views
-
191
Downloads
-
3
Citations
-
 Abstract Abstract
 PDF PDF
- Use of immune checkpoint inhibitors (ICIs) in hepatocellular carcinoma (HCC) has been partially successful. However, most HCC patients do not respond to immunotherapy. HCC has been shown to induce several immune suppressor mechanisms in patients. These suppressor mechanisms include involvement of myeloid-derived suppressor cells, regulatory T-cells, functionally impaired dendritic cells (DCs), neutrophils, monocytes, and tumor associated macrophages. The accumulation of immunosuppressive cells may lead to an immunosuppressive tumor microenvironment as well as the dense fibrotic stroma which may contribute to immune tolerance. Our laboratory has been investigating different cellular mechanisms of immune suppression in HCC patients. In vitro as well as in vivo studies have demonstrated that abrogation of the suppressor cells enhances or unmasks tumor-specific antitumor immune responses. Two or three effective systemic therapies including ICIs and/or molecular targeted therapies and the addition of innovative combination therapies targeting immune suppressor cells may lead to increased immune recognition with a greater tumor response. We reviewed the literature for the latest research on immune suppressor cells in HCC, and here we provide a comprehensive summary of the recent studies in this field.
-
Citations
Citations to this article as recorded by  - Higher Number of Tumor-Infiltrating PD-L1+ Cells Is Related to Better Response to Multikinase Inhibitors in Hepatocellular Carcinoma
Ji Won Han, Ji Hoon Kim, Dong Hyun Kim, Jeong Won Jang, Si Hyun Bae, Jong Young Choi, Seung Kew Yoon, Jaegyoon Ahn, Hyun Yang, Pil Soo Sung
Diagnostics.2023; 13(8): 1453. CrossRef - Immune checkpoint inhibitors in HCC: Cellular, molecular and systemic data
Uasim Harkus, Miriam Wankell, Pranavan Palamuthusingam, Craig McFarlane, Lionel Hebbard
Seminars in Cancer Biology.2022; 86: 799. CrossRef - Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
Pil Soo Sung
Clinical and Molecular Hepatology.2022; 28(3): 333. CrossRef
- Tenofovir and Entecavir Have Similar Renal Adverse Events on Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization
-
Young Youn Cho, Young Hwan Choi, Su Jong Yu, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
-
J Liver Cancer. 2019;19(2):128-135. Published online September 30, 2019
-
DOI: https://doi.org/10.17998/jlc.19.2.128
-
-
3,822
Views
-
47
Downloads
-
1
Citation
-
 Abstract Abstract
 PDF PDF
- Background/Aim
s: Tenofovir disoproxil fumarate (TDF) is potentially nephrotoxic in chronic hepatitis B patients. Hepatocellular carcinoma (HCC) patients treated using transarterial chemoembolization (TACE) are at an increased risk of renal injury. The aim of this study was to determine whether TDF is associated with more renal adverse events than entecavir (ETV) in HCC patients treated with TACE.
Methods
In this retrospective single-center study, we selected 53 HCC patients who were treated with TDF from January 2012 to July 2013 and had their first TACE procedure in the same period. These patients were matched by age and sex to patients treated with ETV.
Results
There were no significant differences in baseline characteristics, including HCC factors, and nephrotoxic drug use, between the two groups. The median follow-up period was 17.0 and 20.0 months for the TDF and ETV groups, respectively. There was no difference during the follow-up period between the TDF and ETV groups in the increase in creatinine over 0.5 mg/dL (17.0% and 17.0%, P=1.00, respectively) and the decrease in eGFR over 25% (43.4% and 41.5%, P=0.84, respectively). Multivariate analysis revealed that Child-Pugh class over B (hazard ratio [HR], 7.30; 95% confidence interval [CI] 2.79-19.10; P<0.01) was associated with increase in creatinine, and Child-Pugh class over B (HR, 82.74; 95% CI 12.31-555.83; P<0.01) and Barcelona-Clinic Liver Cancer stage over B (HR, 14.93; 95% CI 1.60-139.51; P=0.02) were associated with decrease in eGFR.
Conclusions
TDF has comparable safety to that of ETV for HCC patients undergoing TACE.
-
Citations
Citations to this article as recorded by  - Big Data Information under Proportional Hazard Mathematical Model in Analysis of Hepatitis B Virus Infection Data of Patients with Interventional Liver Cancer through Antiviral Therapy of Entecavir
Yichi Zhang, Shuai Zhao, Han Ding, Xiaoling Song, Huijie Miao, Xuya Cui, Jian Wang, Bing Han, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef
- Hepatocellular Carcinoma with Segmental Portal Vein Invasion Exhibiting a Complete Response after Transarterial Radioembolization
-
Jun Sik Yoon, Su Jong Yu, Yun Bin Lee, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
-
J Liver Cancer. 2019;19(2):159-164. Published online September 30, 2019
-
DOI: https://doi.org/10.17998/jlc.19.2.159
-
-
 Abstract Abstract
 PDF PDF
- The treatment options available for patients with hepatocellular carcinoma (HCC) with portal vein invasion (PVI) include sorafenib, transarterial radioembolization (TARE), radiation therapy (RT), transarterial chemoembolization with RT, and proton beam irradiation. Herein, we present a case of HCC with segmental PVI that was managed via TARE. The patient had a 4 cm HCC that invaded the segment VIII portal vein branch without extrahepatic spread. Liver function was Child-Pugh grade A, and performance status was good. TARE was performed without any adverse events, and a radiological complete response (CR) was achieved. Thereafter, the patient was followed-up every 3-6 months without any further treatment, and the CR was maintained for >3 years. Therefore, TARE may be a useful alternative therapeutic option for patients with HCC exhibiting segmental PVI.
- A Case of Hepatocellular Carcinoma with Recurrent Peritoneal Metastasis after Hepatectomy Who Showed Complete Response by Surgical Resection
-
Hyo Young Lee, Jeong-Hoon Lee, Joon Yeul Nam, Young Chang, Hyeki Cho, Young Youn Cho, Eung Ju Cho, Su Jong Yu, Yoon Jun Kim, Jung-Hwan Yoon
-
J Liver Cancer. 2017;17(2):153-157. Published online September 30, 2017
-
DOI: https://doi.org/10.17998/jlc.17.2.153
-
-
 Abstract Abstract
 PDF PDF
- Recurrence of hepatocellular carcinoma (HCC) after hepatic resection is quite common. Peritoneal
recurrence has been considered incurable status and related to poor prognosis. Although
peritoneal metastasectomy is a therapeutic option for some selected patients with a few
peritoneal metastasis, the indication and therapeutic effect has not been clear. We report a
case
of a 61-year-old man achieving complete remission of recurrent peritoneal metastasis after
repeated surgical resection by a multidisciplinary approach. Peritoneal metastasectomy might
be a therapeutic option for selected patients with localized oligonodular peritoneal metastasis.
|